Probiotics represent a potential innovative therapeutic approach in the treatment of depression, acting in a targeted and simultaneous way on specific metabolic disorders often associated with dysbiosis of the microbiota in the intestine. The PRO-DEMET randomised clinical trial evaluated the efficacy of a probiotic formulation composed of selected strains, with the objective of investigating the influence on depressive state and on certain metabolic parameters.
The study is distinguished by a solid protocol, from a methodological point of view, on the basis of which a pilot investigation and a subsequent main study were developed (Gawlik-Kotelnicka et al., 2021; 2023; 2024), the results of which provide relevant indications for optimising probiotic interventions in the treatment of depression, particularly in subjects with specific metabolic profiles (1,2,3).
PRO-DEMET, description of the study
The research ‘The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation and Oxidative Stress Parameters and Faecal Microbiota in Patients With Depression Depending on Metabolic Syndrome Comorbidity‘ (PRO-DEMET) is an intervention study (Randomized Controlled Trial, RCT) carried out in Poland in the city of Łódź (pronounced ‘Wootch’) between December 2020 and May 2023. The study was regularly entered in the US clinical trials database. (4)
The probiotic preparation used contains the lactic bacteria Lactobacillus helveticus Rosell®-52 and Bifidobacterium longum Rosell®-175. The choice of these strains is based on preliminary evidence emerging from previous intervention studies and experiments on animal models, which have highlighted their potential effectiveness in modulating the microbiota-gut-brain axis in both healthy subjects and patients suffering from depression.
The experiment aimed to identify a potential safe and easy-to-use therapeutic option, to be used as additional therapy in a sub-population of depressed patients who respond only partially to pharmacological treatment. The basic hypothesis is that the comorbidity of depression and metabolic syndrome increases the probability of the therapeutic effect of probiotic supplementation in the case of depressive and anxious symptoms through the increase in the diversity of the microbiota in the faeces and therefore the synthesis of short-chain fatty acids (SCFA).
Research protocol
The study is of a prospective, randomised type, double-blind and controlled with four arms, in parallel groups, which included 200 participants for a duration of 20 weeks. The parameters evaluated included:
- psychological and quality of life variables (levels of depression, anxiety, stress and quality of life);
- anthropometric and cardiovascular parameters (blood pressure, body mass index and waist circumference);
- haematological and biochemical indicators (white blood cell count, serum levels of C-reactive protein, HDL cholesterol, triglycerides and fasting blood glucose);
intestinal microbiota profiles (composition and levels of specific faecal metabolites); and finally - systemic markers of inflammation and oxidative stress in the serum.
The selection of patients, carried out in compliance with the pre-established eligibility criteria, took place through 1:1 randomisation, in blind, based on the presence or absence of metabolic syndrome. This allowed the formation of four distinct groups, named as follows:
- PRO-DMS. Probiotics + depression + metabolic syndrome;
- PLC-DMS. Placebo + depression + metabolic syndrome;
- PRO-D. Probiotics + depression;
- PLC-D. Placebo + depression.
The intervention provided for the maintenance of normal eating and physical activity habits by the participants, in order to obtain a reliable baseline assessment. The groups treated with probiotics differed from the placebo group exclusively for the presence of active microbial strains in the capsules, identical in excipients and external appearance. The outcomes were analysed on the basis of data collected through self-administered questionnaires and the analysis of biological samples (blood and faeces) provided by the participants.
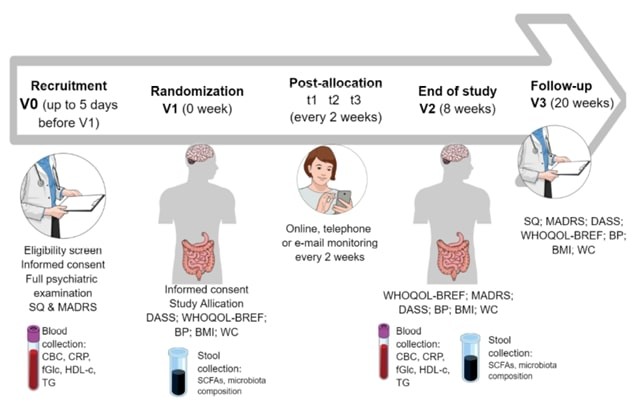
Fig. 1 – Overview of the execution of the PRO-DEMET study (source: Gawlik-Kotelnicka et al., 2021). (5)
Results of the pilot study
The pilot study made it possible to verify the validity of the initial hypothesis, with a view to its extension to a complete RCT. However, some limitations emerged, mainly linked to the selection of participants, hindered by the pandemic and by the distribution of participants affected by metabolic syndrome in the reference groups, not homogeneous due to its low prevalence in the overall sample (27%).
The composition of the groups did not show significant differences in terms of sex, age, psychiatric diagnosis or pharmacological treatments. Even the basic eating habits proved to be quite similar, with the exception of the consumption of dairy products and eggs, which however did not produce significantly different effects either on the psychometric scores or on the metabolic and inflammatory markers at the beginning of the intervention.
The results of the pilot study were used to optimise the design of the main study, with the aim of increasing statistical significance and reducing potential sources of distortion in the effect of the treatment, such as selection bias or selective dropout (e.g. number of participants with metabolic syndrome).
Results of the main study
The main study involved 116 patients, recruited from primary care clinics and psychiatric outpatient clinics. The inclusion criteria included a diagnosis of depressive disorder and a score equal to or greater than 13 on the Montgomery-Åsberg Depression Rating Scale (MADRS). (6) The researchers highlighted some interesting differences in the group treated with probiotics:
- a slight reduction in the level of lymphocytes, the only inflammatory marker to show a significant variation, and
- an improvement in the neurovegetative domain (for example sleep and appetite), indicative of a clinically significant change (MCID), albeit minimal.
Probiotics, while not proving sufficient as a monotherapy for the reduction of depressive symptoms, have proven promising as an integrative support to other pharmacological or psychological treatments. It is important to emphasise that the study was conducted in an outpatient clinical population with depression (tertiary prevention), a different context compared to other research carried out on healthy subjects or with depression in comorbidity (primary and secondary prevention).
Neither diet nor levels of physical activity significantly influenced the effectiveness of the probiotic intervention, which nevertheless proved safe and well tolerated at the dose used (3 × 10⁹ colony forming units, CFU). This dosage is appropriate for similar interventions, while leaving room for potential optimisations based on individual characteristics – genetic, symptomatological or metabolic – such as the selection of specific strains, their combinations or a different duration of treatment. Furthermore, the presence of more marked metabolic abnormalities (for example overweight or hepatic steatosis) could attenuate the psychometric benefits, perhaps due to an underlying dysbiosis that the probiotics aim precisely to correct.
Provisional conclusions
The PRO-DEMET study has brought innovative results on the use of probiotics in patients with depressive symptoms. Probiotic formulations can be used as an effective complementary treatment for depressive disorders. Comorbidity with other factors such as obesity or hepatic steatosis can influence the effectiveness of probiotic treatments for depression, anxiety and stress. However, further research on the details of such interventions is essential, also taking into account further studies on other types of subjects (e.g. healthy people with depressive symptoms).
The first results, although limited, offer interesting opportunities to direct research towards the development of precise formulations of probiotic microorganisms capable of improving mental health. A higher number of participants, and greater consideration of disturbing elements (e.g. percentage of body fat, more accurate markers of the state of inflammation), must be included in future studies.
The obstacles provided by the European Commission are certainly not useful to consumers, who see restricted possibilities of choosing safe foods capable of exerting a positive effect on the intestinal microbiota and, by reflection, on the entire state of health, including mental health.
Probiotics and health, the European obstacle
The European Commission – with a decision devoid of legal value, which conflicts with the interpretations offered by various Member States (e.g. Italy, Spain) – has associated the term ‘probiotic‘ with a health claim, the use of which is bound to a specific prior authorisation pursuant to the Nutrition and Health Claims Regulation (EC) No 1924/06, NHCR. This decision, as already discussed in depth on Food Times (Dongo, 2025):
- expresses a technical error, as it is motivated by reference to the definition of probiotic provided by FAO (Food and Agriculture Organization) and WHO (World Health Organization) which refers the word ‘health’ to the host organism and not to the individual (7);
- forces operators in the sector to face enormous burdens to carry out disproportionate analyses (double-blind tests against placebo on healthy individuals), or to give up informing consumers about the actual nature of the substances (probiotics), which instead finds exact definition and test criteria in the joint document of FAO and WHO;
- severely penalises investments in research and development in a sector that has already shown great potential in improving people’s health, favouring the balance (eubiosis) of the intestinal microbiota;
- unjustly limits consumer rights to identify products that actually contain probiotic ingredients, prejudicing their possibilities of making informed purchasing choices.
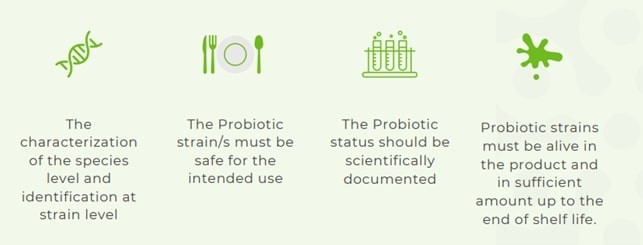
Fig. 2 – Requirements suggested by IPA Europe for the use of the term ‘probiotic’ (source: IPA Europe, 2025) (8)
International Probiotics Organisation (IPA) Europe has in turn published a manifesto where it is emphasised that the term ‘probiotic’ is commonly associated with a category of food ingredients that contain microorganisms (generally, lactic bacteria and/or yeasts) that are alive and viable. Substances that meet the international criteria established to define ‘probiotics‘ must therefore be able to be named as such, to allow their identification, without this implying any suggestion of health benefits.
Dario Dongo and Andrea Adelmo Della Penna
References
(1) Gawlik-Kotelnicka O. et al. (2021) The Influence of Probiotic Supplementation on Depressive Symptoms, Inflammation, and Oxidative Stress Parameters and Fecal Microbiota in Patients with Depression Depending on Metabolic Syndrome Comorbidity—PRO-DEMET Randomized Study Protocol. Journal of Clinical Medicine 10-1342, https://doi.org/10.3390/jcm10071342
(2) Gawlik-Kotelnicka O. et al. (2023) PRO-DEMET Randomized Controlled Trial on Probiotics in Depression—Pilot Study Results. Nutrients 15-1400, https://doi.org/10.3390/nu15061400
(3) Gawlik-Kotelnicka O. et al. (2024) Metabolic Status Influences Probiotic Efficacy for Depression—PRO-DEMET Randomized Clinical Trial Results. Nutrients 16-1389, https://doi.org/10.3390/nu16091389
(4) National Library of Medicine (U.S.). (2021, February 19). The influence of probiotic supplementation on depressive symptoms, inflammation, oxidative stress parameters and fecal microbiota in patients with depression depending on metabolic syndrome comorbidity. ClinicalTrials.gov. Identifier NCT04756544. https://clinicaltrials.gov/ct2/show/NCT04756544
(5) Si illustrano gli acronimi utilizzati nell’immagine: BMI – body mass index; BP – blood pressure; CBC – complete blood count; CRP – C-reactive protein; DASS – Depression, Anxiety, Stress Scale; fGlc – fasting glucose; HDL-c – high-density protein cholesterol; MADRS – Montgomery-Åsberg Depression Rating Scale; SCFAs – short-chain fatty acids; SQ – study questionnaire; TG – triglycerides; WC – waist circumference; WHOQOL-BREF – The World Health Organization quality of life-BREF
(6) Il MADRS è un questionario diagnostico utilizzato dai professionisti della salute mentale per misurare la gravità degli episodi depressivi in soggetti con disturbi dell’umore
(7) FAO/WHO. (2001). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report. http://www.fao.org/3/a-a0512e.pdf
(8) International Probiotics Association (IPA) Europe (2025). A holistic approach to probiotics in the EU for informed consumers and a sustainable food industry. https://tinyurl.com/4wzvsw8k
Bibliography
- Cerdó, T., García-Santos, J. A., Bermúdez, M. G., & Campoy, C. (2019). The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients, 11(3), 635. https://doi.org/10.3390/nu11030635
- Dinan, T. G., & Cryan, J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterology Clinics of North America, 46(1), 77-89. https://doi.org/10.1016/j.gtc.2016.09.007
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., Morelli, L., Canani, R. B., Flint, H. J., Salminen, S., Calder, P. C., & Sanders, M. E. (2014). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506-514. https://doi.org/10.1038/nrgastro.2014.66
- Ng, Q. X., Peters, C., Ho, C. Y. X., Lim, D. Y., & Yeo, W. S. (2018). A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of Affective Disorders, 228, 13-19. https://doi.org/10.1016/j.jad.2017.11.063
- O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., & Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research, 277, 32-48. https://doi.org/10.1016/j.bbr.2014.07.027
- Sarkar, A., Lehto, S. M., Harty, S., Dinan, T. G., Cryan, J. F., & Burnet, P. W. (2016). Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends in Neurosciences, 39(11), 763-781. https://doi.org/10.1016/j.tins.2016.09.002
- Wallace, C. J. K., & Milev, R. (2017). The effects of probiotics on depressive symptoms in humans: A systematic review. Annals of General Psychiatry, 16, 14. https://doi.org/10.1186/s12991-017-0138-2









