Zeolite, the bubbling stone. Swedish mineralogist Alex F. Cronstedt, in 1756, coined this term-from the Greek ‘boil’(zein) and ‘stone’(lithos)-to describe a family of minerals that, when subjected to heating, appear to boil due to the rapid loss of the water they contain.
Zeolites are microporous minerals with large structural cavities. Forty-six types exist in nature, and it is in Italy that those with the highest cation exchange capacity are mined. With very interesting applications in agriculture and animal husbandry-as well as human health-to date underestimated.
Zeolite in agriculture
The use of natural zeolite in agriculture has ancient traditions in some areas of Japan, where clinoptilolite and mordenite are still used for soil pH control.
Further applications related to the nutritional aspects of plants arise from zeolite’s ability to act as a slow-release agent in the soil and thus improve the retention of nitrogen compounds and nutrients in general.
Useful for soil fertility
Ion exchange plays a key role in agriculture and is also a valuable support in organic farming. The addition of zeolite to the soil results in increased exchange capacity toward nutrients, and ultimately improved soil fertility. In fact, zeolites added to the soil, especially those rich in K+, release potassium gradually, depending on the needs of the plants.
Such materials can also be ‘loaded’ with organic matrix nitrogen compounds, which in turn are released slowly, with reduced dispersion in the environment and more efficient uptake by crops. Staying in the soil then allows it to act as a ‘flywheel’ for positively charged nutrients over time.
A natural plant defense
Zeolite is also useful as an adjuvant in crop defense due to a threefold action:
– Dehydrating agent on the surface of leaves and fruits. So as to prevent the establishment of a microclimate suitable for the development of fungal pathogens,
– protection toward insects, with a discrete intensity mechanical action protective barrier on leaves and fruits,
– invigorating (for stimulating and strengthening plant defenses), by virtue of the well-defined crystal structure with an anionic lattice that generates special electric fields. Which in turn stimulate immune defenses and promote healing of lesions–on branches, fruits and leaves–caused by biotic and abiotic factors.
Animal husbandry, the benefits on animals and in slurry management
In animal husbandry, zeolites are successfully used to feed animals and manage slurry.
Feed supplementation with zeolite (at a rate of 5-6%) is associated with increased animal weight and reduced incidence of various diseases and conditions. Clinoptilolite in particular reduces ammonium and urea toxicity in animals by absorbing volatile nitrogen components.
The physiological effects of natural zeolites are attributed to their high exchange capacity and the high selectivity shown for species-such as NH4+, Pb2+, Cd2+, Cu2+, Cs+ and other cations-that can accumulate in animal tissues.
Zeolites can then find use in effluent management by soaking in sewage. Thus, it is possible to load the nitrogenous organic fractions for later distribution on the soil. This results in a reduced residual pollutant load of animal manure while making a slow-release nitrogen fertilizer.
Zeolites in the removal of pollutants from wastewater
Another application of zeolites is in wastewater purification. Studies on the cation exchange properties of zeolites began as early as the 1950s (Colella, 1996), with some practical applications in environmental protection. Therefore, the well-defined crystalline structure of zeolites, thanks to the anionic lattice that generates special electric fields, enables the removal of some polluting cations from wastewater.
Applications of zeolites as cation exchangers in the field of water purification mainly concern the removal of
– NH4+ from municipal and industrial wastewater,
– Heavy metals from industrial wastewater,
– Radionuclides from nuclear power plant wastewater.
Zeolites and personal well-being
Under certain circumstances and with specific goals, zeolite can be activated for medical or dietary supplement purposes, and more generally for personal well-being. By virtue of its strong cation exchange capacity, the mineral is thus suitable for use in some medical devices and dietary supplements, with adjuvant functions in some therapies i.e., as a detoxifier and antioxidant.
Activation is a process to increase porosity or to increase surface area by reducing particle size. This provides more channels capable of binding toxins, radicals and metals faster and more stably, increasing their activity. In fact, starting from a size of about 3 m2/g of zeolite, even 1000 m2/g is reached after activation.
The detox function
The special characteristics of activated clinoptilolite zeolite consist of its ability to bind free radicals, heavy metals, ammonium ion and toxins at the intestinal level, removing them from the body, activities described in the definition drawn up by the European Nomenclature of Medical Devices.
Natural silica materials-including clinoptilotite zeolite-reveal multiple biological activities and are already successfully used as an adjuvant in various therapies, with favorable results on overall health status.
Donato Ferrucci and Dario Dongo
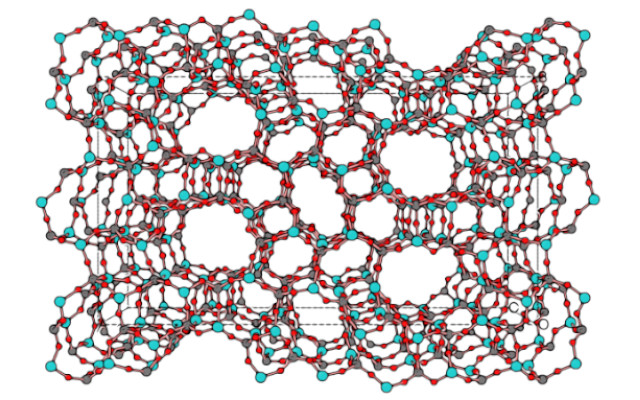
Cover image: Lee, J. K., Turrina, A., Zhu, L., Seo. S., Zhang, D. Cox, P. A., Wright, P. A., Qiu, S. and Hong, S. B.An Aluminophosphate Molecular Sieve with 36 Crystallographically Distinct Tetrahedral Sites.Angew Chem Int Ed. 53 7480-7483 (2014). DOI: 10.1002/anie.201402495
Notes
(1) Zeolites are microporous minerals that belong to the class of tectosilicates. They have a very open crystal structure, with cavities ranging in volume from 30 to 50 percent of the volume of the entire crystal. In their natural state, the cavities and channels are occupied by Na+, K+, Ca2+ ions (normally exchangeable) and water molecules. About 60 species of zeolitic minerals have been identified in nature.
The chemical formula is: (Me (m/z))・[Alm・Sin・O2(m+n)]・qH2O, with Me representing a metal cation foreign to the structure (Li+, Na+, K+,Ca2+, Sr2+, Ba2+, Mg2+, etc.).
Of interest are the cation exchange and adsorption capacity. These result from the particular structure and host cations. This determines that the cations, which are present in the channels and cavities to balance the negative charge of the lattice resulting from the presence of aluminum in tetrahedral coordination, are rather weakly bound to the anionic scaffold of the zeolite and can therefore be ‘exchanged’ with their surroundings.
Bibliography
Anaclelio S. Production of advanced ceramics with unconventional technologies. PhD thesis in materials and structural engineering. University of Naples Federico II. Faculty of Engineering.12.11.09 http://www.agribionotizie.it/la-zeolite/, https://www.chimicamo.org/chimica-generale/zeoliti.html, http://www.chimdocet.it/solido/file11c.htm